
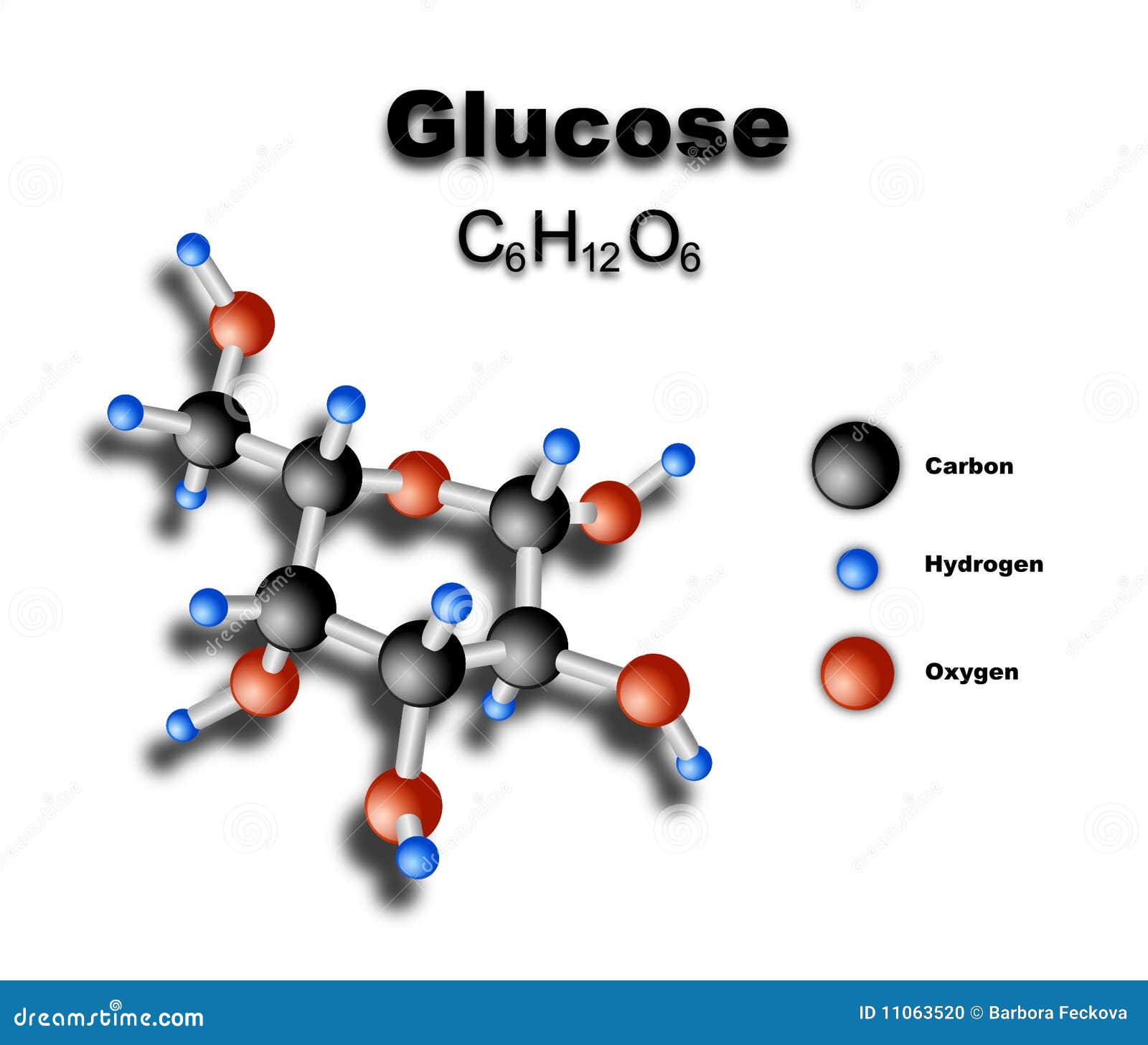
Simulation studies suggested distinct conformational changes for the GLUT4 domains in the ATP substrate-bound form and favor a constricted behavior for the transport channel. Principal component analysis suggested a clockwise movement for the domains in the apo form, whereas ATP substrate-bound form induced an anti-clockwise rotation. Simulation studies with the substrate bound form proposed a stable state of GLUT4 with glucose, which can be a substrate-occluded state of the transporter. Apo form simulation analysis revealed an extracellular open conformation of GLUT4 in the membrane favoring easy exofacial binding of substrate.
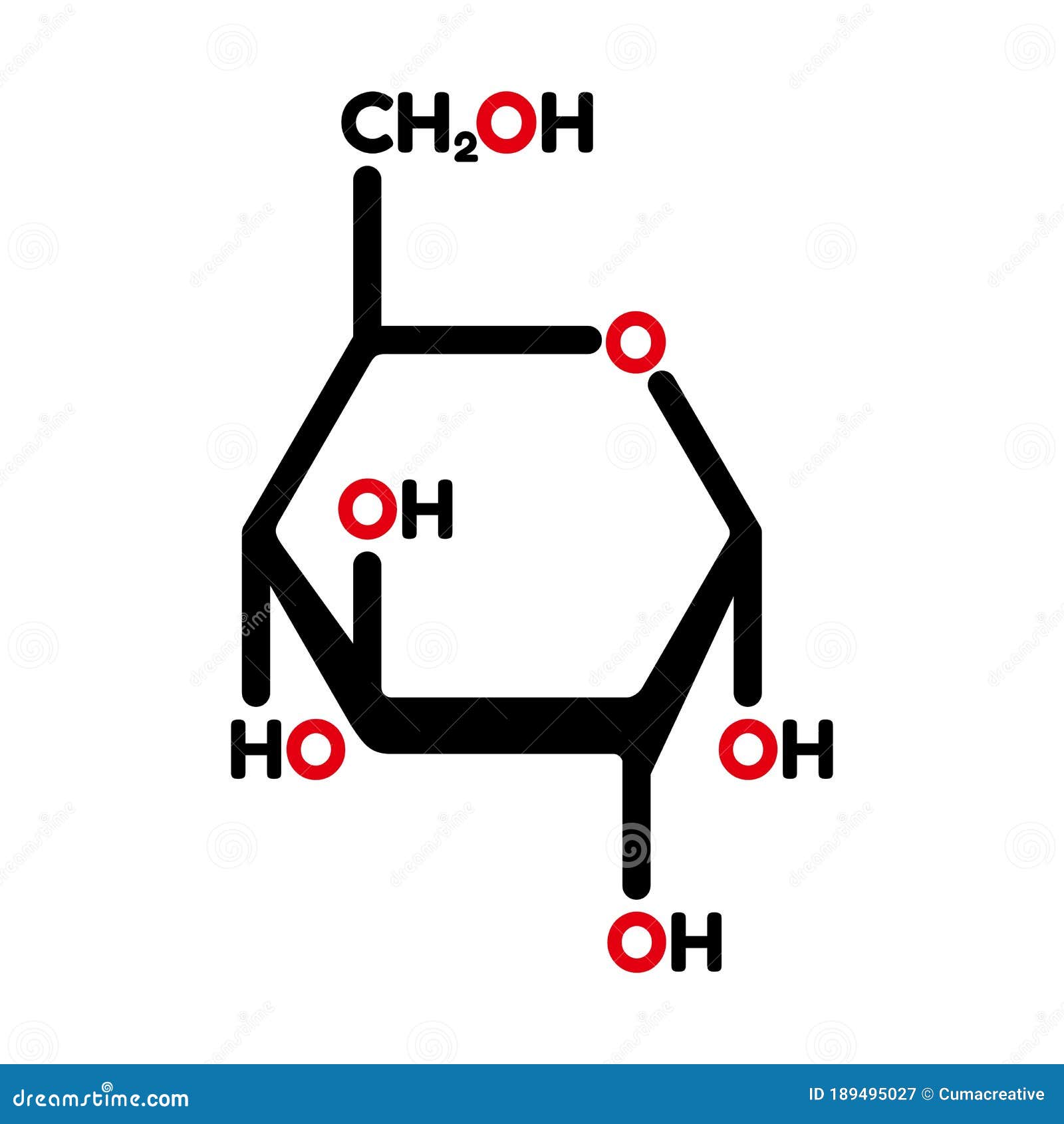
In the present study, the dynamic behavior of GLUT4 in a membrane environment was analyzed using three forms of GLUT4 (apo, substrate and ATP-substrate bound states).


 0 kommentar(er)
0 kommentar(er)
